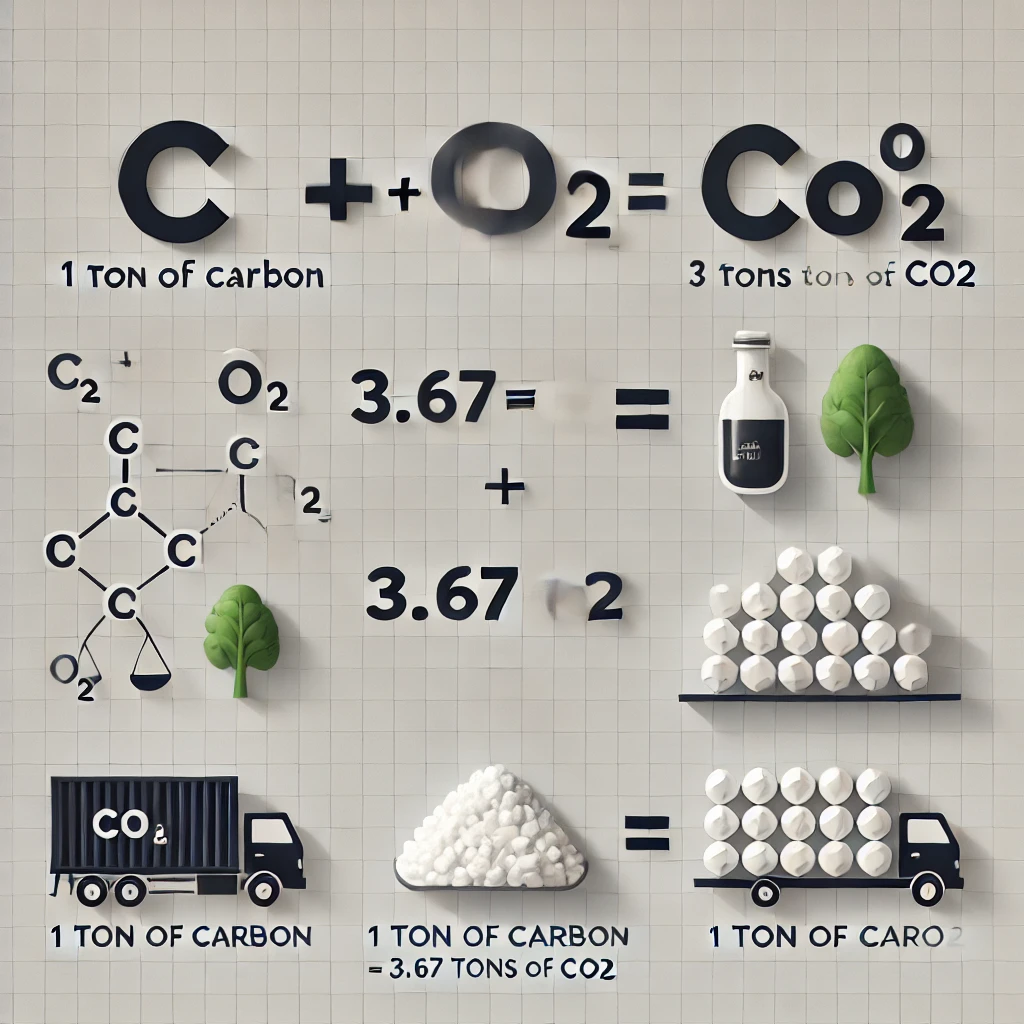
From atomic mass calculations:
- CO₂ molecular weight = C (12) + O₂ (16×2) = 44
- Carbon’s proportion in CO₂ = 12/44 ≈ 0.273 (~27.3%)
Therefore,
12 grams of carbon is contained in 44 grams of carbon.
1 gram of carbon is contained in 44/12 grams of carbon.
1 ton of carbon is contained in (44/12) x 1 ton of carbon.
So, 1 ton of carbon = (44/12) × 1 = 3.67 tons of CO₂